Specialized assays to further characterize your compounds
Aside from our standard pharmacology assays for potency, efficacy, and affinity determination, we have developed specialized assays to bridge the gap between in vitro and in vivo data. Such as kon/koff determination, chemotaxis, biomarkers production (insulin, IFNγ, TNFα…).
Kinetics of competitive binding for kon/koff determination
Direct kinetics of ligand-receptor interactions are not Always taken into account in the optimization of a drug’s pharmacokinetics properties. However, several studies have shown that slow dissociation rates in vitro can be associated to long-acting effects of a compound in vivo. This important parameter can be measured as the rate at which the complex dissociates (expressed as koff or dissociative half-life) into the free ligand and its target protein. The dissociative half-life has been connected to improved clinical efficacy in a large number of marketed drugs.
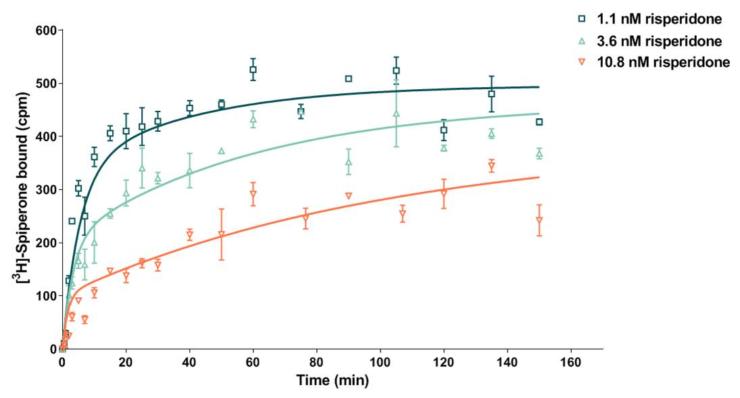
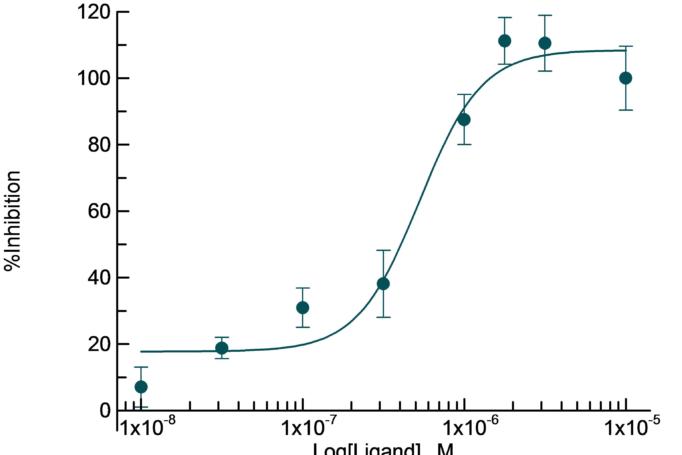
Determination of the kon/koff for the potent and selective D2 receptor antagonist Risperidone, by monitoring the association of [3H]-Spiperone in the presence of increasing concentrations of compound.
| Risperidone binding to D2 receptor | |
| kon | 4.4 107 M-1 min-1 |
| koff | 0.036 min-1 |
Cell migration assay (recombinant and primary cells)
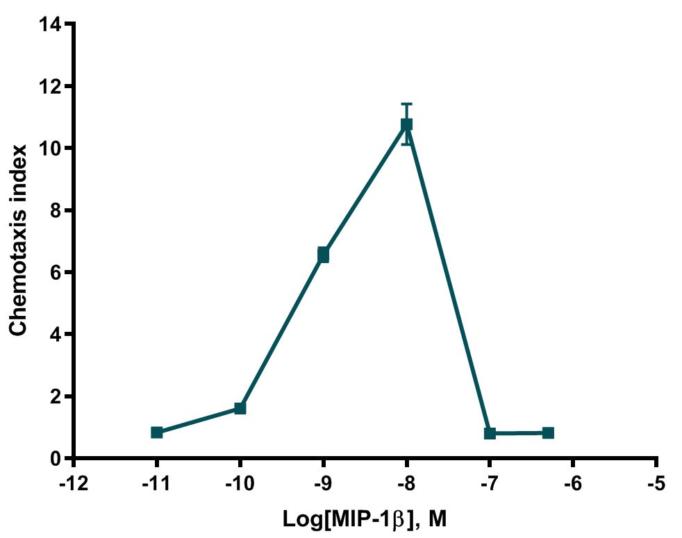
Cell migration is a fundamental function of normal cellular processes, including embryonic development, angiogenesis, wound healing, immune response, and inflammation. One of these processes, leukocyte extravasation, is crucial for appropriate and effective immune response. Leukocytes normally exist in a resting state as they circulate through the body. However, upon interaction with small molecules known as chemoattractants, they rapidly respond with endothelial adhesion followed by emigration from the vasculature and chemotaxis to the site of inflammation.
CHO-K1 cells stably expressing recombinant human CCR5 receptor migrate in response to MIP-1β. Cell migration is effectively and dose-dependently blocked by a CCR5 receptor small molecule antagonist.
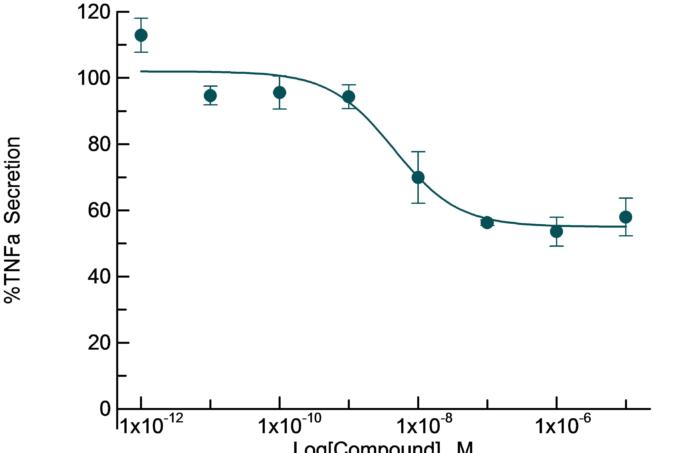
Cytokine release from LPS-stimulated human PBMC
The human LPS-stimulated peripheral blood mononuclear cells (PBMC) is a good preliminary profiling assay to evaluate a compound’s effect on inflammatory cytokine production.
Inhibitory effect of dexamethasone on pro-inflammatory TNFα cytokine release from LPS-stimulated human PBMC
Glucose-Stimulated Insulin Secretion (GSIS) from isolated rat pancreatic β-islets
Type 2 Diabetes (T2D) is a devastating worldwide public health problem. Drug therapies are available to address this disease, but the common occurrence of side-effects, or lack of efficacy after long-term treatment causes concern. Moreover, the growing patient population suffering from T2D and associated metabolic diseases creates a demand for new therapies.
Testing candidate compounds for insulin secretion on freshly-isolated rat pancreatic islets allows to quickly and cost-effectively screen for those likely to perform better before moving to in vivo models.
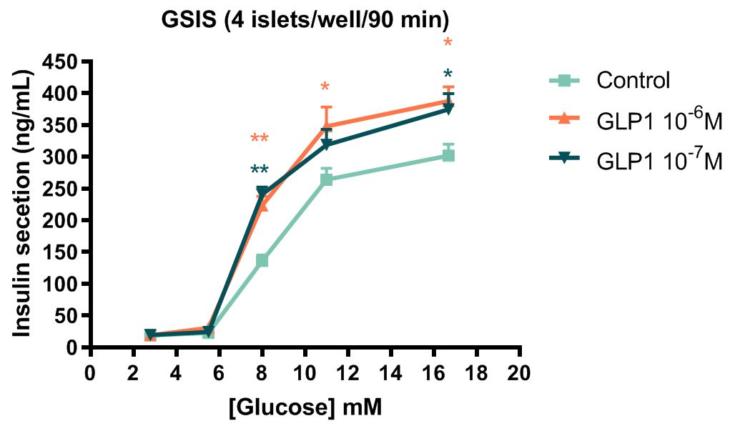
Datas are presented as mean +/- SEM. Statistical analysis are ANOVA test followed by a DUNETT test.
** p<0.01 vs control
* p<0.05 vs control
Freshly-isolated rat pancreatic islets were incubated with increasing concentrations of glucose, in presence or absence of GLP-1(7-36) peptide. Cell supernatant was harvested and insulin was quantified by HTRF.
Off-the-shelf and custom-based enzymatic assays
Enzymes represent a fast-growing class of targets in the drug discovery industry, as principal targets for screening like kinases or epigenetic modulators, or as potential safety issues when assessing off-target effects. consequently we have recently put our scintific expertise to the development and validation of several enzymatic assasy including kinases, MAO, COMT, PDE, COX or AchE.
Steroid 5-α-Reductase (SRD5a) assay cutsom-developed for a specific application. CHO-K1 cells expressing recombiannt human SRD5a are used to induce testosterone conversion to androstanolone. This activity is dose-dependently inhibitied by Linolenic Acid and intact testosterone is quantified by HTRF.